PiMedical – Medical Solution
PiMedical is the solution for customers with regulatory requirements in the GMP area.
MES systems and machine terminals (HMI) used in medical sector must comply with the regulatory guidelines according to GAMP5.
We fulfil the organisational requirements by delivering the software development process through all phases according to our software quality plan (SQP). MES PiSolutions also covers the technical and functional requirements with the PiMedical modules.
We are the ideal partner if you are considering any of the following as a priority:
- Documentation for software development according to GAMP5
- Software Quality Plan – description of procedure for internal software development
- Tests: Software Module Tests, System Integration Tests, Unit Tests)
- Software release
- Change management and corrective actions
- Life cycle management
- Software security
- System backup and restore
- Disaster Recovery
Electronic records
- User administration
- Audit trail
- Versioning
- Pharmaceutically relevant parameters
- Batch records
- Input validation
Reasons behind our success
Process understanding
We speak the same language as the machine and the manufacturer, thus distinguishing ourselves from the classic MES provider. We would be happy to demonstrate how our system could work for you.
Interface knowledge
Based on our experience, we have knowledge and expertise in a wide range of different interfaces. This enables us to successfully integrate our products in a variety of industries, regardless of machine type, or control system.
Customer needs
Our expertise in the field of MES, Automation Engineering and Software Engineering, helps us respond to our customers individual requirements, achieved by the knowledge and experience of our long-standing employees.
PiMedical – the medical modules
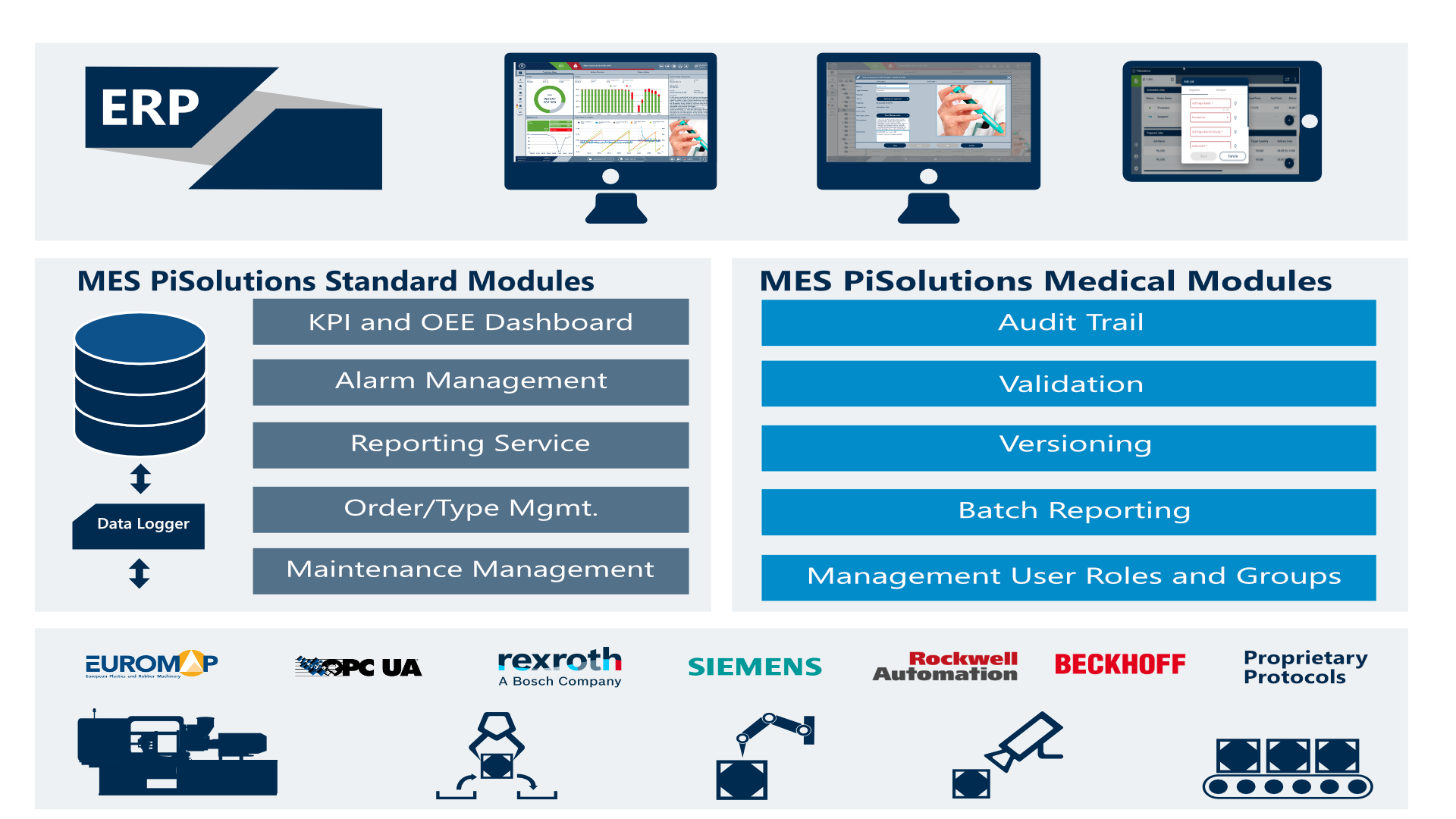
Audit Trail
The audit trail is used to record and log all actions and events according to GMP CFR Part 11 performed on the system. This data is stored in the database and can be displayed for a freely definable period of time. The data can be used for analysis and production monitoring.
Validation
The electronic signature is intended for tracking changes or assigning the user who made the changes an integral part of the Medical Solution. Changes, e.g. of pharmaceutically relevant parameters, can only be made if the user logs in again beforehand.
Versioning
Management of product types and their parameters as well as their distribution to the controllers. In addition, the historical storage of product types including versioning is included in the functional range.
Type parameters can be changed temporarily in a running job. For a new order with the same product type the type parameters are loaded from the product type management. The temporarily changed type parameters are overwritten with the default values again.
Batch Reporting
Evaluations of the documents required for the duty of proof and export to PDF or printer.
Management User Roles and Groups
The user management offers the possibility to create a separate user account for each person. Each user must be assigned to one of the user groups. One or more roles are assigned to the user groups. These roles group the actual access rights to the individual resources.
To use and interact with the user management, users must log in. This is especially important for audit trails where user interactions must be logged with user names.
The user management supports Windows users (Active Directory) as well as local users.
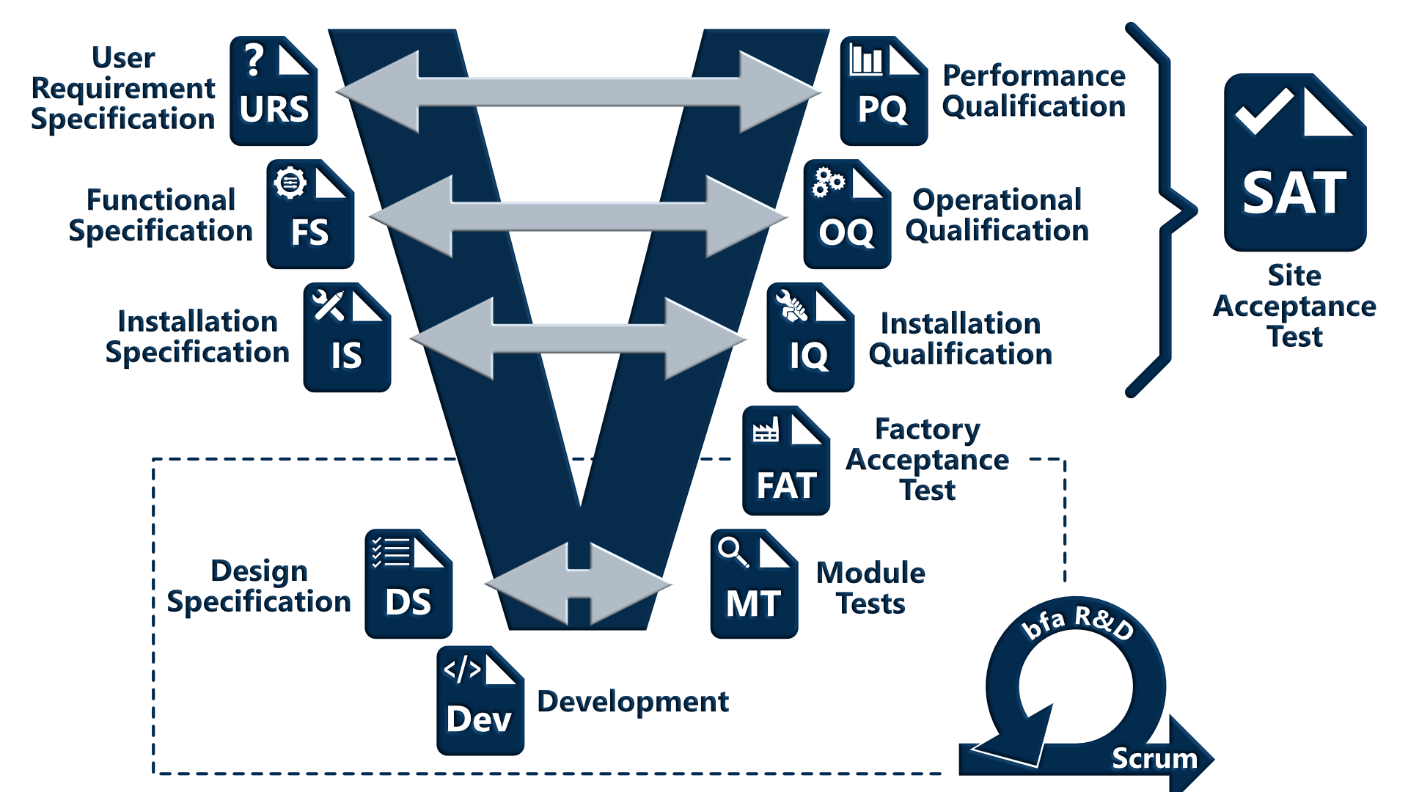
Software Development Process
The software development process is based on the V-Model and contains elements of agile software
development (e.g. daily scrum meetings).
We are there for you.
Please send us an email or call us on +41 44 806 64 64.
